Abstract
Introduction
Double or triple hit high-Grade B cell Lymphoma (HGBL), which appeared on the 4th revised edition WHO classification of lymphoid neoplasms in 2016, is a group of agressive mature B-cell lymphomas which morphogically ressemble diffuse large B-cell lymphoma (DLBCL) or with features intermediate between DLBCL and Burkitt lymphoma, that have a MYC (8q24) rearrangement in combination with a BCL2 (18q21) and / or a BCL6 (3q27) rearrangement. There are currently no treatment guidelines for DH/TH HGBL, and the usual chemotherapy leads to few complete remissions. While the whole exome sequencing technology has been used in diffuse large B cell lymphoma, there has been few studies on additional DH/TH HGBL genetic events which would predict the clinical outcome. Here, we report our preliminary results on DH/TH HGBL patients using a targeted DNA capture panel approach (NGS) to identify subgroups with different clinical outcomes among DH/TH HGBL.
Methods
We conducted a retrospective study on 66 patients diagnosed with DH or TH HGBL and treated at Centre Hospitalier Lyon Sud or Centre Léon Bérard starting in 2013. We collected the medical information regarding the treatment, time to response to treatment or relapse, progression free survival, and overall survival. In addition, we performed a preliminary NGS analysis on the most recent samples using SophiaGenetics LYS panel (54 genes) in Centre Léon Bérard.
Results
The cohort included 30 females and 36 males, with a median age of 65 (IQR: 56.25-72.75). Among them, 32 patients were MYC/BCL2 DH (48.49%), 14 were MYC/BCL6 DH (21.21%) and 20 were TH (30.30%). Nine patients had B symptoms (9/62 14.52%). The Ann Arbor stage was high (III-IV) (59/66, 89.39%). The WHO Performance Status was low (0-1-2) for most of the patients (52/59, 88.14%). The majority of them presented with an IPI above to 2 (39/51, 76.47%) and an elevated LDH level (48/62, 77.42). The Ki67 positivity was above 70% (52/62, 83.87%) in most of the patients and above 90% (27/62, 43.55%) in nearly half of them. The median follow-up time was 11 months (IQR: 6-37.75) for the cohort.
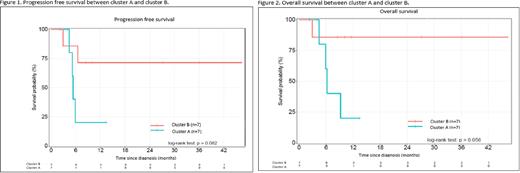
The major finding of the NGS analysis was the identification of 2 clusters based on the TP53/CDKN2A mutational status. The first group included patients with mutually exclusive TP53 or CDKN2A alterations (cluster A, n=7) while the second group had no alteration in these two genes (cluster B, n=7). TP53 alteration consisted exclusively in gene mutations while we found loss of heterozygoty or point mutation for CDKN2A. The median progression free survival (PFS) (Figure 1) and overall survival (OS) (Figure 2) were 5.39 months (IQR: 6.51-NA) and 6.18 month (IQR: 5.88-NA) for group A respectively, and were not achieved for cluster B. The median follow-up was 5.88 months (IQR: 4.24-7.69) for cluster A and 11.56 months (IQR: 9.3-31.6) for cluster B, the difference being due to the higher mortality rate in cluster A.
Conclusion Our preliminary results indicate a strong negative prognostic impact of either CDKN2A or TP53 gene alterations on the PFS and OS in HGBL DH/TH patients. Although the difference was not statistically significant between clusters with only 14 patients having NGS results, we plan to include other samples to further consolidate the data.
More importantly, the biological basis for the difference between clusters would be in favour of the alteration of the ARF-TP53 tumor suppressive pathway as major contributor to survival since one of our sample had only a point mutation in ARF start codon.
Taken together, our results suggest that routine NGS analysis of HGBL DH/TH patients may offer new perspectives on patient stratification and possibly new classification and therapeutic opportunities such as MDM2 or BCL2 inhibitors, in combination with conventional chemotherapy and need to be confirmed in a larger population.
Disclosures
Sesques:Chugai, Novartis, and Kite/Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal